TECHNOLOGY
MBR
(Membrane Bio-Reactor)
HEM Pharma can produce probiotics, LBP (Live Biotherapeutic Product), and cultured extracts that can be used as cosmetic raw materials by using MBR (Membrane Bio-Reactor), which requires technical skills and expertise. The MBR culture method is a new method that combines bioreactor and membrane filtration, and produces 10 to 50 times higher concentration of cells than conventional batch or fed-batch culture. In MBR fermentation, which requires high technology in design and operation, microbial culture raw materials are continuously supplied, and various fermented substances generated in the fermentation process are continuously filtered through a membrane, so high-concentration culture is possible. The MBR used by our company is a system to which a tubular type filtration membrane that mimics the intestinal environment is applied, and the produced probiotics and LBP have high stability and survival rate, which has the advantage of excellent acid resistance to survive in the stomach acid in the human body when consumers take it.
MBR Process Summary Chart
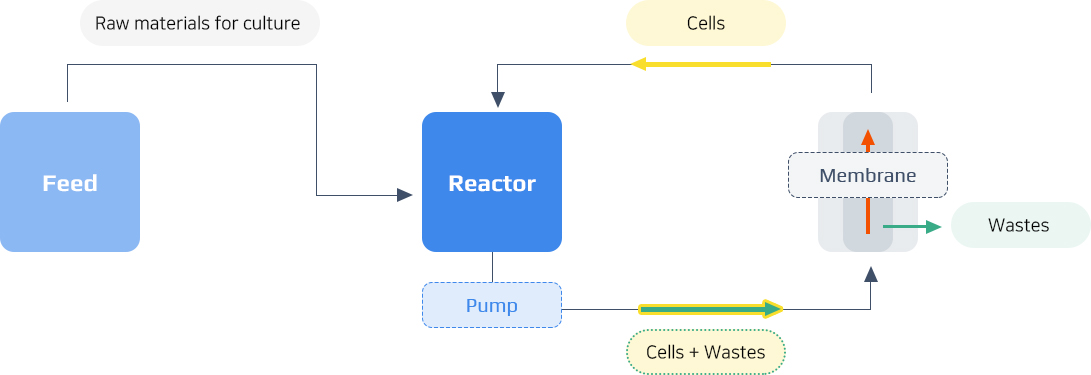
- Inoculation
- Cultivation
- Concentration
- Harvest
MBR Process
-
MBR culture / Filtration / Extraction / Concentration
-
Drying of a Cells
-
Mixing / Pulverization
-
Quality inspection (microorganism test)
-
Product packaging and shipment
MBR Strong Points
Comparison of technology
HEM Pharma's MBR system is a next-generation culture system that compensates for the shortcomings of the process used by existing overseas CMO companies.
| Name | MBR | W** | U** | B** | C** |
|---|---|---|---|---|---|
| Country | HEM, Korea | Germany | USA | France | Swiss |
| High pressure culture | O | X | X | X | X |
| Automation | O | X | X | X | X |
| Oxygen exposure | Low | Low | High | High | High |
| Culture method | Continuous type | Continuous type | Batch type | Batch type | Batch type |
| LBP separation technology | Membrane | Membrane | Centrifuge | Centrifuge | Centrifuge |
Economic feasibility
After simultaneous culture and freeze-drying, the final production yield showed a high production yield with 69 ~116% of MBR-producing strains compared to 15-31% of normal cultured strains.
| Cultivations | Culture broth | Mixtures of anti-freezing agents and cells | Freeze-dried powders | |||
|---|---|---|---|---|---|---|
| Vol (mL) | Yield (%) | Weight (g) | Yield (%) | Weight (g) | Acc. / yield (%) | |
| Comparison of different cultivation at Jar fermenter cultures. | ||||||
| Jar 1st | 3500 | 100% | 289 | 16% | 65.5 | 15% |
| Jar 2nd | 3400 | 100% | 134.62 | 65% | 26.34 | |
| Comparison of different cells from three consecutive MBR cultures. | ||||||
| MBR 1st | 18600 | 100% | 1359 | 148% | 257 | 70% |
| MBR 2nd | 6900 | 100% | 631 | 79% | 133 | 92% |
| MBR 2nd* | 3900 | 100% | 631 | 79% | 133 | |
| MBR 3rd | 16100 | 100% | 1267 | 81% | 322.3 | 69% |
After inoculation to collection, 2nd and 3rd culture and recovery are possible by injecting additional culture materials after the end of the process.
- Cultivation
- Concentration
- Harvest
Efficiency
With the increase in culture efficiency, production per unit volume increases.
| Compare items | Batch culture / fed-batch culture | MBR culture |
|---|---|---|
| Growth, OD600nm | 5~20 | >100 |
| Number of bacteria, cell/mL | 5×109 ~ 2×1010 | > 1×1011 |
Functionality
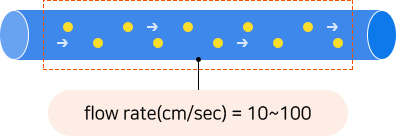
As the intestinal tract are mimicked, and microorganisms pass through a circular water tube with a diameter of 4mm and a length of 1m(10~100cm/sec), it is possible to conduct a culture in a state similar to that of the human microbiome and high cell stability raw materials are produced.